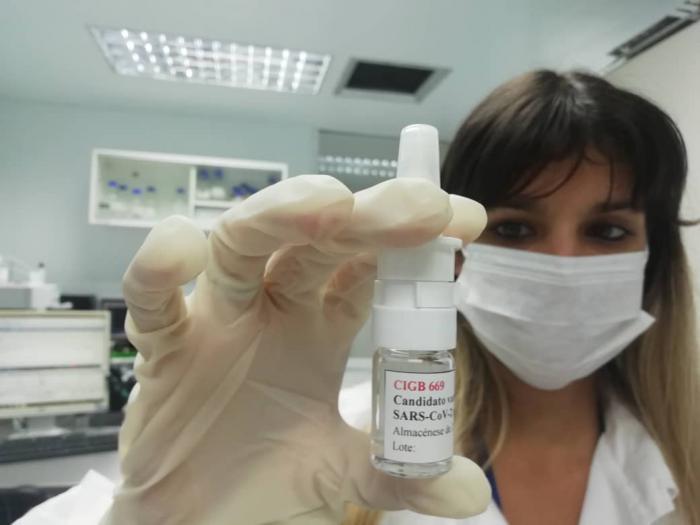
With the Hermanos Ameijeiras Hospital as clinical site, Phase I/II trials of the candidate vaccine Mambisa and the Abdala vaccine began yesterday, in Havana, to evaluate and compare the safety and immunogenicity of the two drugs.
The study, as explained on Cuban television by Dr. Iglermys Figueroa, head of Allergy Services at the institution, involves administrating Mambisa to three groups of recovered COVID patients, and Abdala to one.
She explained that three groups will receive Mambisa to test three different methods of administration, nasal spray, drip or a Cuban device adapted to a syringe, to evaluate which is best, before continuing to the trial’s second phase.
The doctor reiterated that the volunteer subjects are convalescent COVID patients who have developed a certain level of antibodies naturally, and the objective is to reinforce their immunity, to avoid re-infection.
Regular evaluation by Cuba's Center for the State Control of Medicines, Equipment and Medical Devices (Cecmed), which approved the study, will continue during this process, and on the first day focused its attention on compliance with good clinical practices.
In this regard, Dr. Diadelis Remírez stressed that the entire traceability process of the clinical trial is being reviewed, and the services of clinical laboratories and pharmacies will be investigated in greater depth, as well, with a view toward certifying them in the future.
The trial includes 120 volunteers from the municipality of Centro Habana, between 19 and 80 years of age, who were discharged from the hospital at least two months, following a SARS-COV2 infection.















