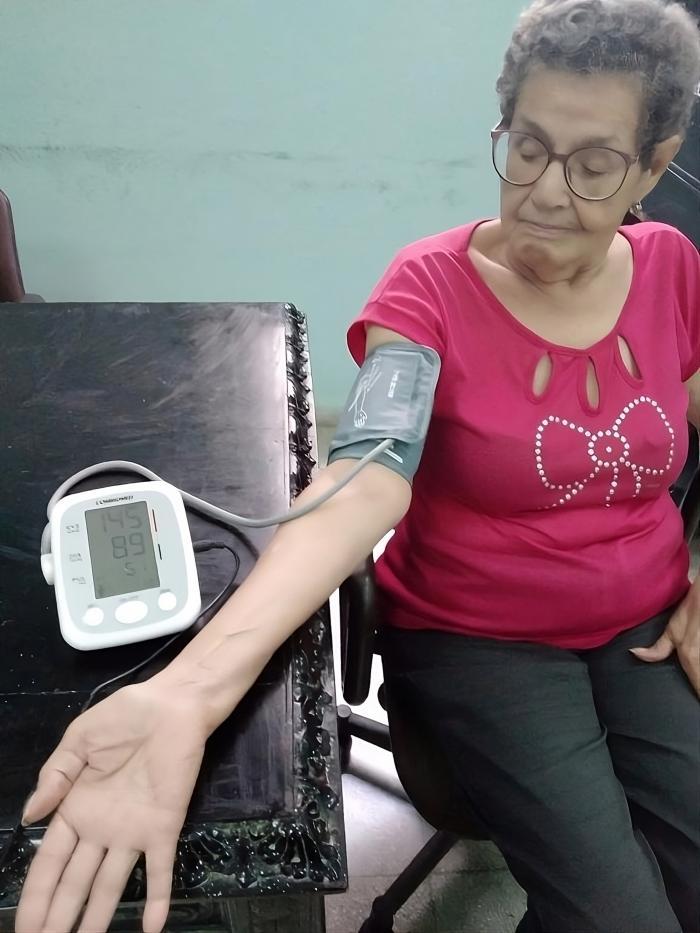
Cuba took a significant step forward in its efforts to prevent and control arterial hypertension in its population, with the recent clinical validation of the Hipermax BF automatic sphygmomanometers, developed by the company Combiomed Digital Medical Technology.
To achieve this certification, during the last three years a clinical trial called Validation Study for the clinical use of the Hipermax BF automatic blood pressure measuring device in the general population was carried out, promoted by the Institute of Cardiology and Cardiovascular Surgery (ICCV), of the Ministry of Public Health (Minsap), under the leadership of the State Center for Control of Medicines, Equipment and Devices (Cecmed).
Dr. Yamilé Valdés González, a specialist in Internal Medicine and vice-president of the National Technical Advisory Commission on Hypertension of the Minsap, explained to Granma that the research, aimed at achieving the stated objective, included a broad sample of people aged 12 and older.
"After a rigorous review by international experts, the results that endorse the granting of this condition have just been published in the academic journal Journal of Human Hypertension. Likewise, they are visible in the Cuban Public Registry of Clinical Trials."
According to Dr. Valdés González, since 2020 the World Health Organization (WHO) recommends the exclusive use of validated upper arm sphygmomanometers, whenever possible and in accordance with the socioeconomic particularities of each country.
Regarding the importance of having this credential, the member of the Board of the World Hypertension League emphasized that Hipermax BF equipment now has a guarantee of safety and high precision.
"This gives added value to this Cuban product in the international market, thus increasing marketing opportunities in the Latin American and Caribbean region, where there is a limited supply of certified devices."
She pointed out that only about 15% of the automatic sphygmomanometers marketed in the world have passed the clinical validation tests, making the one manufactured by Combiomed even more meritorious.
"To be included in the international lists of devices that have that status, it is essential to complete the required clinical trial and publish the results in a public registry or in a high-visibility scientific journal," said Dr. Valdés González.
Cuba is one of the few countries in the Americas region that produces this automatic equipment. However, it did not have the capacity to conduct clinical validation studies, which are reserved for highly specialized laboratories in industrialized countries and are expensive.
"This research was carried out thanks to the technical cooperation of Minsap and the Pan American Health Organization, through the Hearts in the Americas initiative, with the participation of experts from the University of Tasmania, in Australia; the University of Calgary and Alberta, in Canada, and the University of Medical Sciences of Havana."
Introduced since 2015, today there are more than 5,000 Hipermax-BF devices available in the National Health System, and work is underway to increase production, taking into account the high demand.
"There is a general belief that conventional sphygmomanometers are better than automatic ones, and this is not the case. The former are based on the use of the auscultatory method with the support of a stethoscope, so they depend on the skills and training of the medical staff to listen to the noises associated with the passage of the bloodstream through the vascular system.
"In relation to the automatic ones, they use the oscillometric method with which it is possible to detect, through a transducer, the highest frequency of oscillation of the vessel walls and then, by means of mathematical algorithms, the systolic (maximum) and diastolic (minimum) pressures of the patients are calculated, without human intervention, as a possible source of error. They are therefore more accurate."
In both variants, she stressed, it is vital to ensure adequate preparation of the patient and to comply with the established procedure for measuring blood pressure.
She praised the technical leadership assumed by Dr. Damaris Hernández Véliz, assistant professor and researcher and director of the Institute of Cardiology and Cardiovascular Surgery, in the entire process linked to the design and execution of the validation clinical trials.
SUPPORT FOR CUBAN MEDICAL TECHNOLOGY
Having a group of highly qualified professionals and technologists, committed to the country and endowed with an admirable innovative capacity, has been the key to the significant contributions made by the company Combiomed Digital Medical Technology to the medical care of Cubans.
Engineer Arlem Fernández Sigler, general director of the company, which belongs to the BioCubaFarma Business Group, said that in the last three decades Combiomed has introduced more than 35,000 medical devices and equipment in hospitals and polyclinics in all provinces.
"Among them, eight generations of electrocardiographs for resting tests stand out, including the family of Cardiocid t50/s100/d200 equipment; the three generations of ambulatory electrocardiographic recording systems or Holter tests, in addition to defibrillators, equipment for patient monitoring and pulse oximeters and digital sphygmomanometers, such as the Hipermax BF."
"Having been able to manufacture it, in a quantity of more than 5,000, is a technological achievement, but the fact that it has been validated and works with the required international standards represents a great result of Cuban science", he said.















