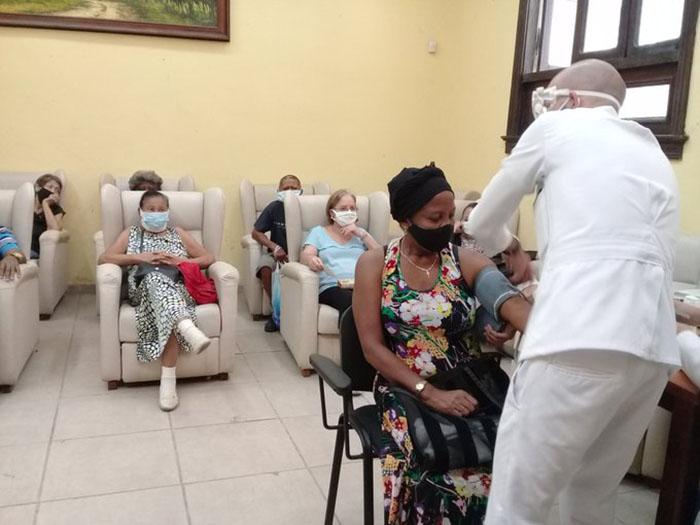
The first week of phase III clinical trials of Soberana 02, with volunteers between 19 and 80 years of age, was completed "satisfactorily, in strict compliance with the established protocol and best clinical practices," reported the state enterprise group BioCubaFarma, on Facebook.
The posted message emphasized that this initial stage "of piloting and adjustment of processes, closes with 4,478 individuals vaccinated, and only mild side effects in some subjects observed, such as pain at the injection site in the first 24 hours following administration of the candidate, and general malaise."
According to this report, as of March 12, 40 vaccination centers were functioning, a number which should increase over the next few days in the eight Havana municipalities included in the study.
On its official Twitter account, the Finlay Vaccine Institute noted that, during the recently begun phase, Soberana 02 "continues to demonstrate its safety."
Havana's Provincial Defense Council reported that during each day that has elapsed, almost all volunteers scheduled were vaccinated, thanks to the work of community family doctor and nurse offices.
Phase III clinical trials of Soberana 02, the first Latin American anti-covid-19 candidate vaccine to reach this stage, will include some 44,000 persons living in the capital, in the municipalities of Plaza, Playa, Diez de Octubre, Centro Habana, Marianao, Habana Vieja, Cerro and La Lisa, and are focused on evaluating the efficacy of the vaccine’s immunogen.















